Accelerating Evidence Synthesis: an Introduction to Rapid Reviews following the Cochrane Rapid Reviews Methods Guidance
Event Details
Background: Rapid reviews are a time-sensitive and efficient approach to evidence synthesis used in healthcare decision-making. As the demand for timely and reliable evidence increases, traditional systematic reviews may
Event Details
Background:
Rapid reviews are a time-sensitive and efficient approach to evidence synthesis used in healthcare decision-making. As the demand for timely and reliable evidence increases, traditional systematic reviews may not always be feasible due to their resource-intensive and lengthy nature. Although rapid reviews may not offer the same level of comprehensiveness as systematic reviews, they provide valuable insights to inform decision-makers in time-critical situations, offering a balanced approach between timeliness and quality of evidence. As the demand for rapid and relevant evidence continues to grow, the adoption of rapid reviews has become increasingly prevalent in healthcare research and policy domains.
Understanding how to conduct rapid reviews following best practices is essential for ensuring the reliability and relevance of the evidence synthesized. The Cochrane Rapid Reviews Methods Group has been a pioneering force in the development of rigorous rapid review methodologies. They aim to disseminate their guidance and expertise to researchers, policymakers, and decision-makers interested in utilizing evidence-informed rapid review methods. By adhering to these robust practices, stakeholders can efficiently produce evidence that is timely, trustworthy, and aligned with the needs of healthcare decision-making. Embracing this evidence synthesis approach will contribute to more informed and evidence-based decision-making in urgent and time-sensitive healthcare contexts, ultimately improving patient care and health outcomes.
Aim:
To provide an introduction to rapid review methods for interventions of effectiveness following the updated Cochrane Rapid Reviews Methods Guidance.
Objectives:
To equip participants with the knowledge to conduct efficient and high-quality rapid reviews. Based on updated guidance developed by the Cochrane Rapid Reviews Methods Group, participants will learn various strategies to accelerate evidence synthesis, optimize team composition, and tips for present findings in a concise and impactful manner to decision-makers.
Learning outcomes:
Upon completing this course, participants will gain the following skills:
- Ability to gasp the fundamental concept of rapid reviews and their critical role in healthcare decision-making processes.
- Ability to identify scenarios where rapid reviews are appropriate and understand the significance of involving Knowledge Users (KUs) in the rapid review process.
- Knowledge of what is involved in developing efficient and tailored literature search strategies specifically crafted for rapid reviews.
- Learning how to build effective teams for rapid reviews and insight into recommended techniques to streamline essential processes, including study selection, risk of bias assessment, data extraction, and evidence synthesis phases.
- Understanding recommended best practices, such as the significance of reporting guidance, for optimizing the effectiveness of rapid reviews.
These learning outcomes will help participants develop a comprehensive understanding of rapid reviews and equip them with the practical skills and knowledge necessary to conduct them effectively in the healthcare decision-making context.
Teaching Strategies:
The workshop will consist of a mixture of short presentations and practical exercises/ breakout group discussions.
Places: 30 places available for individuals who are resident on the island of Ireland
Fee: General €50; Student €25
Skill level: Introductory
Prerequisites: Some experience with systematic reviews
Target Audience:
Healthcare professionals, academics, researchers, decision makers, librarians, information specialists, and Evidence Synthesis Ireland fellows and teaching faculty who would like to learn more about rapid reviews.
Facilitators
Dr Chantelle Garritty, Co-Convener Cochrane Rapid Reviews Methods Group; Manager, Global Health and Guidelines Division, Public Health Agency of Canada; Adjunct Professor, School of Epidemiology and Public Health, University of Ottawa Cochrane Central Executive Team
Dr Barbara Nussbaumer-Streit, Co-Director Cochrane Austria, University for Continuing Education Krems, Austria; Co-Convenor Rapid Reviews Methods Group
Dr Candyce Hamel, Senior Epidemiologist, Canadian Association of Radiologists
Dr Ursula Griebler, Senior research associate at the Department for Clinical Epidemiology and Evaluation, University for Continuing Education Krems, Austria; Associate Convenor Cochrane Rapid Reviews Methods Group
Course content
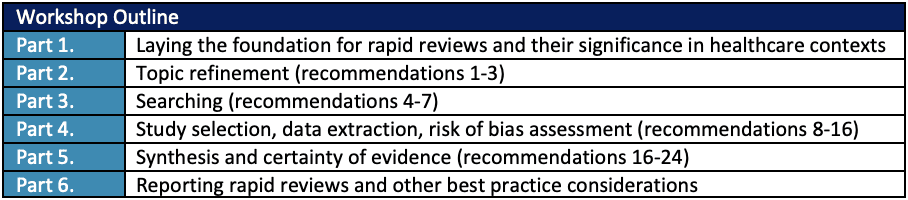
*If your type of ticket is sold out, please join the waitlist or contact us at esi@universityofgalway.ie
more
Additional Information
Click HereRegistration
Click HereTime
(Wednesday) 1:00 pm - 4:00 pm
Location
ONLINE
