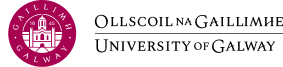Making more of
Evidence Syntheses
Evidence Synthesis Ireland (ESI), which hosts Cochrane Ireland, aims to make evidence syntheses more usable in every sense of the word – better designed, conducted and reported, more useable for decision-makers and more usable within health care policy and clinical practice decision making across the island of Ireland and beyond.
What We Do
Provide Training
We provide education for researchers, clinicians, the public and policymakers who conduct or use evidence syntheses.
Build Capacity
We build capacity in planning, doing and sharing evidence syntheses through Fellowships, workshops and scholarships.
Advance Knowledge
We advance the way we plan, do and share the results of evidence synthesis by finding out how to do these steps better.






Upcoming training
Our training streams include qualitative evidence synthesis, scoping reviews and rapid reviews, Cochrane methodology, mixed methods and more
january 2026
Event Details
This workshop will be held over four mornings and provides authors, at the beginning of the systematic review process, with a clear understanding of how systematic reviews of the effects
Event Details
This workshop will be held over four mornings and provides authors, at the beginning of the systematic review process, with a clear understanding of how systematic reviews of the effects of interventions are planned and conducted. It offers an insight into the development of a protocol, introducing participants to methodology, search methods, data extraction, risk of bias assessment and meta-analysis. While the focus is on systematic reviews of the effects of interventions, the principles discussed are likely to be useful to inform steps and approaches in other types of reviews.
Date: 22nd, 23rd, 29th & 30th January 2026
Time: 10.00am – 1.00pm
Places: 25 available for individuals who are resident on the island of Ireland
Fee: General €150; Student €80
Skill level: Introductory
Target Audience: Healthcare professionals, academics, researchers, policy makers and other decision makers, and Evidence Synthesis Ireland fellows who have identified a systematic review of effects of interventions topic and are ready to begin working on their protocol.
Prerequisites: A basic knowledge of health research. Interested in learning more on the methods of a systematic review of effects of interventions.
Teaching strategies: The workshop will consist of a mixture of short presentations led by members of the Evidence Synthesis Ireland and Cochrane Ireland teaching faculty, covering each of the stages of developing a systematic review protocol, small group activities and plenary discussions, providing participants with the opportunity to develop and refine their protocol. Although focusing on systematic reviews of the effects of interventions, the principles are likely to apply to other review types. This course will include blended learning with two months free access to Cochrane Interactive Learning self-directed learning modules, a number of which will be required study prior to the workshop.
Facilitators
Dr KM Saif-Ur-Rahman, Evidence Synthesis Methods Lead, Evidence Synthesis Ireland & Senior Research Fellow, University of Galway.
Ciara Gleeson, Clinical Specialist Physiotherapist, Respiratory Assessment Unit, St. James’s Hospital, Dublin.
Dr Elayne Ahern, Associate Professor, Department of Psychology, University of Limerick
*If your type of ticket is sold out, please join the waitlist or contact us at esi@universityofgalway.ie
more
Additional Information
Click HereRegistration
Click HereTime
22 (Thursday) 10:00 am - 30 (Friday) 1:00 pm
Location
ONLINE
february 2026
Event Details
To register, CLICK HERE High-quality evidence syntheses are essential for guiding evidence-based healthcare and policy decisions. Yet, an increasing number of methodologically flawed reviews—often single-authored, lacking
Event Details
To register, CLICK HERE
High-quality evidence syntheses are essential for guiding evidence-based healthcare and policy decisions. Yet, an increasing number of methodologically flawed reviews—often single-authored, lacking comprehensive searches, dual screening, proper risk of bias assessment, or certainty-of-evidence evaluation—are being published in peer-reviewed journals. Such practices risk misleading decision-makers, undermining public trust, and diluting the scientific value of evidence synthesis. In this webinar, we will discuss how the peer review and journal editorial process can play a critical role in safeguarding the integrity of evidence synthesis. Drawing on personal and professional experiences, the presenters will outline practical strategies for strengthening peer review and editorial oversight, including enforcing reporting guidelines, improving editorial and reviewer expertise, and exploring the responsible use of artificial intelligence. The session will highlight how rigorous peer review processes can ensure trustworthy syntheses that inform policy, practice, and public understanding.
Speaker:
Dr. David Moher is a senior scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, where he directs the Centre for Journalology. Dr. Moher is also a full Professor, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa. Dr. Moher received an MSc in epidemiology and PhD in clinical epidemiology and biostatistics. Dr. Moher is a fellow of the Royal Society of Canada, and Canadian Academy of Health Sciences. Dr. Moher works in knowledge synthesis; predatory journals; reporting guidelines; and open science.
Dr. KM Saif-Ur-Rahman specialises in evidence synthesis and health research methods, holding the position of Director of Ireland GRADE Network, Evidence Synthesis Methods Lead at Evidence Synthesis Ireland and Cochrane Ireland, Senior Research Fellow at Center for Health Research Methods, University of Galway, and Honorary Research Senior Lecturer at the College of Medicine Nursing and Health Sciences, University of Galway. He holds an MBBS, an MPH in Epidemiology from Bangladesh, and a PhD in Epidemiology from Nagoya University, Japan. Over the past decade, he has led and contributed numerous evidence syntheses in public health, including systematic reviews, rapid reviews, scoping reviews, and evidence gap maps. His portfolio includes more than 130 peer-reviewed publications, with several Cochrane and methodological reviews as lead or senior author. Dr. Saif is also a dedicated mentor and educator, co-supervising PhD students, leading the Evidence-Based Future Healthcare MSc module at University of Galway, and delivering training across Bangladesh, Japan, and Ireland. He serves as Associate Editor for BMC Systematic Reviews, is a member of the GRADE Working Group, and is Vice-Chair of the University of Galway Research Ethics Committee.

more
Registration
CLICK HERETime
(Thursday) 2:00 pm - 3:00 pm
Location
ONLINE
10feb1:00 pm2:30 pmWebinar: Prof. Gordon Guyatt - Core GRADE: Part Two1:00 pm - 2:30 pm ONLINE
Event Details
To register, CLICK HERE GRADE is the world wide standard for rating certainty of evidence in systematic reviews and moving from evidence to recommendations in clinical
Event Details
To register, CLICK HERE
GRADE is the world wide standard for rating certainty of evidence in systematic reviews and moving from evidence to recommendations in clinical practice guidelines. GRADE has, however, become too complicated and the over 50 authoritative GRADE papers are disorganized and increasingly difficult to navigate. In a series of 7 papers published in the BMJ, Gordon Guyatt has led a group of GRADE experts in produce Core GRADE, the essentials of the GRADE process for paired comparisons of interventions. The series provides systematic reviewers and guideline developers with a clear and straightforward guide that includes all they need to produce rigorous systematic reviews and guidelines based on the GRADE process. This second of two webinars will deal with Core GRADE’s approach to inconsistency, indirectness, and moving from evidence to recommendations.
Speaker:
Gordon Guyatt is a McMaster University Distinguished Professor. His work has focused on Evidence-based Medicine. His work has included a key role in the creation of GRADE approach to systematic reviews and practice guidelines, and most recently a series of papers on the essentials of GRADE, Core GRADE in the BMJ. He has published over 1,000 papers many in the top journals and is one of the world’s most cited scientists.

more
Registration
CLICK HERETime
(Tuesday) 1:00 pm - 2:30 pm
Location
ONLINE
Event Details
Background: This series of workshops will provide reviewers, at the beginning of their journey in conducting a Qualitative Evidence Synthesis (QES)
Event Details
Background:
This series of workshops will provide reviewers, at the beginning of their journey in conducting a Qualitative Evidence Synthesis (QES) with a comprehensive overview of the methodology and methods including a Cochrane QES reviews. The series will offer insights into the development of a protocol, introducing participants to the methods of question generation, identification of included studies, data extraction and synthesis, the GRADECERQual assessment of confidence in the findings, and presentation the review for dissemination.
Aim:
The purpose of these workshops is to familarise all participants with the steps involved in conducting a Qualitative Evidence Synthesis.
We have designed these workshops as a companion series to allow attendees to apply what they have learned, enabling them to gain practical experience before progressing to the next stage of the process.
Objective:
The objectives of the workshops are intended to provide participants with an understanding of the methods of QES and equip them with the practical skills needed to conduct such a syntheses effectively.
Workshop 1: will provide an Introduction to QES, including overview. We will explore how you can focus your review question and search for qualitative evidence.
Workshop 2: will highlight the methods of screening and inclusion processes including selecting studies. We will demonstrate how data are extracted for a QES and a Quality Assessment is conducted.
Workshop 3: will delve into choosing a method of synthesis, and demonstrate how to synthesisinse qualitative data and present the findings
Workshop 4: we will illustrate the process of conducting an assessment of confidence in the findings of the QES using the GRADECERQual approach, and explore methods of writing up a review for dissemination.
Learning outcomes:
The learning outcomes for participants in this series should support them to:
- develop an understanding of the principles of QES and its importance in research
- define a research question suitable for QES
- explore methods for systematically searching and selecting relevant qualitative studies
- develop the skills to assess the quality of qualitative research studies
- gain proficiency in data extraction and methods of qualitative data synthesis
- learn how to conduct an assessment of confidence in the findings of a QES
- explore strategies for presenting and reporting the findings of a QES
Teaching strategies:
The workshops will consist of a mixture of short presentations and discussions led by members of the ESI Teaching Faculty, covering each of the methods of a QES. Breakout rooms will be used for small group activities based around worked examples and exercises. These activities will provide participants with the opportunity to discuss and develop their own skills and understanding. Participants will also be provided with a reading list and additional resources.
Places: 30 places available for individuals who are resident on the island of Ireland
Workshop 2 fee: General €40; Student €20
Overall fee for 4 workshops: General €120; Student €60
Skill level: Introductory
Prerequisites: Knowledge of qualitative research methodologies and methods
Target Audience:
Facilitators
Prof. Pauline Meskell, Head of Department of Nursing and Midwifery, University of Limerick
Dr Linda Biesty, Associate Professor (Midwifery), University of Galway
*If your type of ticket is sold out, please join the waitlist or contact us at esi@universityofgalway.ie
more
Additional Information
More informationRegistration
Click HereTime
(Thursday) 10:00 am - 12:00 pm
Location
ONLINE
Event Details
To register, CLICK HERE IN COLLABORATION WITH THE EUROPEAN METHODS WEBINAR SERIES Systematic reviews of randomized controlled trials (RCTs) aim to include all eligible studies in evidence
Event Details
To register, CLICK HERE
IN COLLABORATION WITH THE EUROPEAN METHODS WEBINAR SERIES
Systematic reviews of randomized controlled trials (RCTs) aim to include all eligible studies in evidence synthesis. Unfortunately, it is now clear that many systematic reviews contain so-called problematic studies. A study could be problematic due to research misconduct (including falsification or fabrication of data) or otherwise due to critical errors. Regardless of the reason for the problems, it is important to identify and remove these studies from systematic reviews to prevent them from influencing healthcare decisions. The INSPECT-SR (INveStigating ProblEmatic Clinical Trials in Systematic Reviews) tool has been developed for the purpose of assessing trustworthiness of RCTs in order to identify problematic trials. This interactive webinar will introduce the INSPECT-SR tool by way of application to a published clinical trial. Participants will have the opportunity to use INSPECT-SR to perform trustworthiness checks themselves and will learn how the tool can be incorporated when undertaking a systematic review.
Speaker:
Jack Wilkinson is a Senior Lecturer in Clinical Trials at University of Manchester. His research interests include clinical trials and evidence synthesis, and he is Statistical Editor for Cochrane Gynaecology and Fertility. He has extensive experience in the conduct of integrity investigations for journals and publishers, and he leads the INSPECT-SR project, which developed a tool for the assessment of trial trustworthiness.

more
Registration
CLICK HERETime
(Thursday) 1:00 pm - 2:00 pm
Location
ONLINE
Fellowships
Evidence Synthesis Ireland Fellowship Scheme
The innovative ESI Fellowships give Fellows the opportunity to learn about evidence synthesis, with hands-on experience of how to plan, design, conduct and report an evidence synthesis. Fellows are placed virtually with world-class evidence synthesis centres and review teams in Ireland and internationally, on policy and practice relevant reviews.
Research
Transforming how we understand and share research
We’re committed to improving how we gather, interpret, and share vast amounts of information, a process known as evidence synthesis. Our approach isn’t just about conducting research; it’s about improving the very methods we use to plan, conduct, and share the results of this process.
We work in many different research areas relevant to evidence synthesis and have a particularly strong focus on rapid reviews, knowledge translation and “studies within a review” (SWARs).
“ Reporting on health care topics can rely too much on asking experts or reporting the results of a single study…what would be much more useful to members of the public, especially when it comes to making decisions about their own health, is for people to understand how to situate health claims in the global body of evidence. “